Researchers from Michigan State University are making progress using solar and wind power more efficiently, even when the sun isn’t shining or the wind blowing.
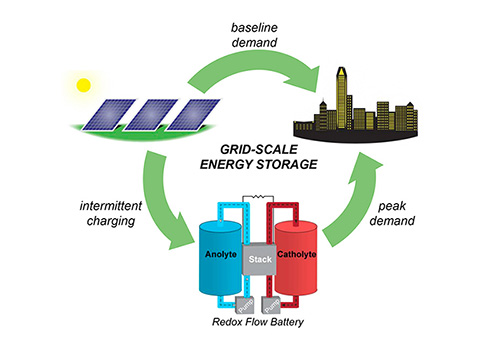
Power sources like solar and wind are intermittent forms of energy generation. Because of this, transitioning completely away from fossil fuels will require a way to store energy that complements intermittent power sources.
The focus is on redox flow batteries, said David P. Hickey, an assistant professor of Chemical Engineering and Materials Science.

“Redox flow batteries can store energy on a much larger scale than conventional lithium ion batteries, because they don’t require precious metals. They are a type of liquid battery that uses special chemicals called redox-active organic molecules.
"These molecules are excellent at holding an electric charge,” Hickey explained, “except they don’t dissolve well in the desired battery fluids. The amount of energy that can be stored is directly related to the solubility of its redox active molecules, so more soluble molecules means higher energy density.”
A team lead by Hickey and Thomas Guarr, cofounder of Jolt Energy Storage Technologies and a professor at the MSU Bioeconomy Institute, recently made a breakthrough in understanding which parts of a molecule’s structure make it more soluble.

Their research findings, C–H···π interactions disrupt electrostatic interactions between non-aqueous electrolytes to increase solubility, have just been published in Nature Chemistry.
“In our study, we looked into a specific type of weak interaction between atoms in the molecules, known as C-H···π interactions. We found that the more of these interactions a molecule has, the better it dissolves in a particular solvent,” Hickey continued.
Hickey and Guarr’s research also shows that even small changes in the molecule's structure can significantly affect its ability to dissolve in the liquid.
“This study is the result of great teamwork by students at both sites, and it provides an important clue to the understanding of a critical property in the design of new materials for next-generation batteries,” Guarr said.

“The discovery brings us a big step closer to an electric grid that does not rely on fossil fuels,” Hickey added.
MSU’s Hickey Group is focused on the design of electroactive small molecules, molecular electronics, and polymer materials for a variety of applications related to energy storage, catalysis, and biosensing. (HickeyLab.org)